Ang rubidyo (Iningles rubidium) mao ang elementong kimiko sa talaang peryodiko nga may simbolo nga Rb ug kaiphan nga atomik 37. Ang rubidyo mao ang puthaw.
| Rubidium
|
37Rb
|
|
|
| Panagway
|
grey white

|
| Kinatibuk-ang mga kinaiya
|
| Ngalan, simbolo, kaiphan
|
rubidium, Rb, 37
|
| Paglitok
|
[[Help:Pronunciation respelling key|roo-Plantilya:Smallcaps all-ee-əm]]
|
| Kategoriyang elemento
|
puthaw
|
| Group, period, block
|
1, 5, s
|
| Gibug-aton sa atomo
|
85.4678(3)
|
| Kontorno sa elektron
|
[Kr] 5s1
2, 8, 18, 8, 1
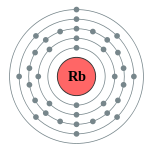 Electron shells of rubidium (2, 8, 18, 8, 1) Electron shells of rubidium (2, 8, 18, 8, 1) |
| History
|
| Pagkadiskobre
|
Robert Bunsen and Gustav Kirchhoff (1861)
|
| First isolation
|
George de Hevesy
|
| Physical properties
|
| Phase
|
magahi
|
| Density (near r.t.)
|
1.532 g·cm−3
|
| Liquid density at m.p.
|
1.46 g·cm−3
|
| Melting point
|
312.46 K, 39.31 °C, 102.76 °F
|
| Boiling point
|
961 K, 688 °C, 1270 °F
|
| Critical point
|
(extrapolated) 2093 K, 16 MPa
|
| Heat of fusion
|
2.19 kJ·mol−1
|
| Heat of vaporization
|
75.77 kJ·mol−1
|
| Molar heat capacity
|
31.060 J·mol−1·K−1
|
| Vapor pressure
|
| P (Pa)
|
1
|
10
|
100
|
1 k
|
10 k
|
100 k
|
| at T (K)
|
434
|
486
|
552
|
641
|
769
|
958
|
|
| Atomic properties
|
| Oxidation states
|
1
(strongly basic oxide)
|
| Electronegativity
|
0.82 (Pauling scale)
|
| Ionization energies
|
1st: 403 kJ·mol−1
|
| 2nd: 2632.1 kJ·mol−1
|
| 3rd: 3859.4 kJ·mol−1
|
| Atomic radius
|
248 pm
|
| Covalent radius
|
220±9 pm
|
| Van der Waals radius
|
303 pm
|
| Miscellanea
|
| Crystal structure
|
body-centered cubic
|
| Magnetic ordering
|
paramagnetic[1]
|
| Electrical resistivity
|
(20 °C) 128 nΩ·m
|
| Thermal conductivity
|
58.2 W·m−1·K−1
|
| Speed of sound (thin rod)
|
(20 °C) 1300 m·s−1
|
| Young's modulus
|
2.4 GPa
|
| Bulk modulus
|
2.5 GPa
|
| Mohs hardness
|
0.3
|
| Brinell hardness
|
0.216 MPa
|
| CAS registry number
|
7440-17-7
|
| Most stable isotopes
|
| Main article: Isotopes of rubidium
|
|
|
| · r
|
Ang mga gi basihan niini
usba
Galeriya sa hulagway
usba


